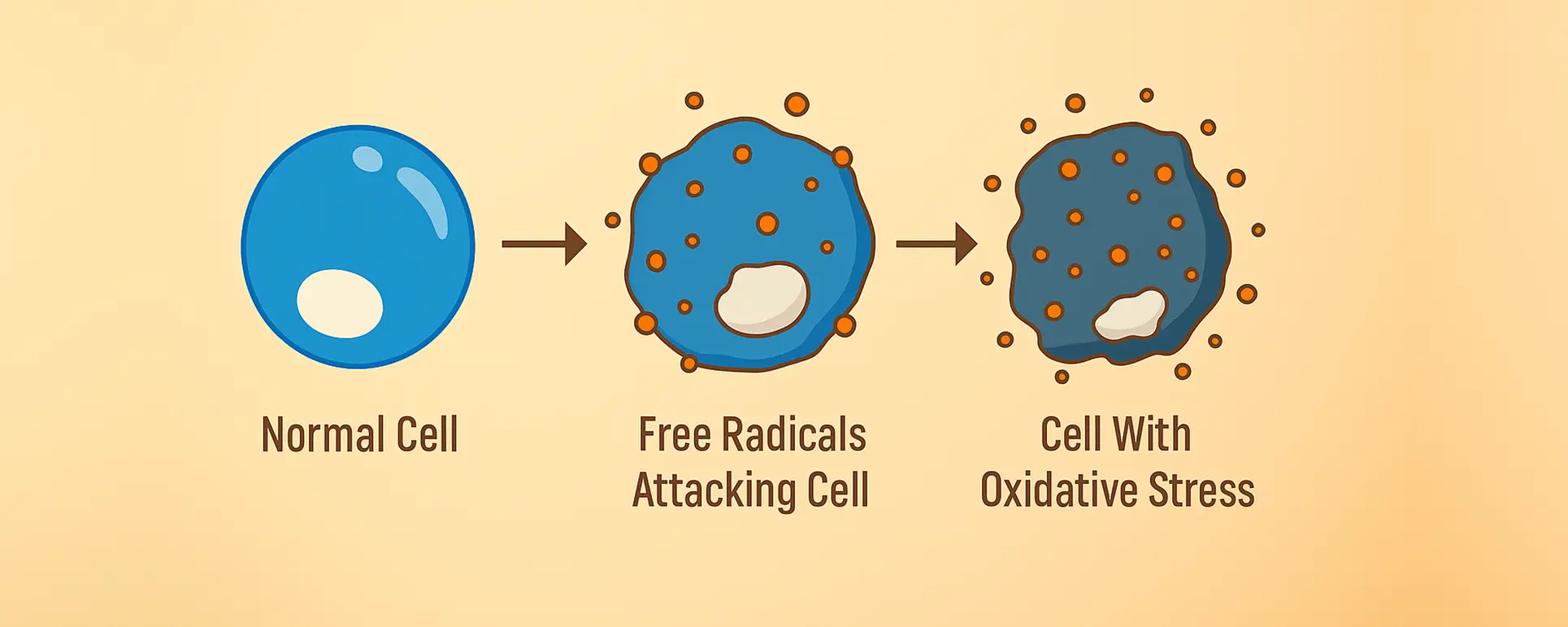
If you’ve ever wondered why cataracts develop as we age—or why even some younger individuals can develop cloudy lenses—oxidative stress plays a major role in the story. This isn’t just about blurry vision or needing stronger glasses. It’s about what’s happening at a cellular level inside your eye’s lens and how small molecular culprits like reactive oxygen species (ROS) can lead to big vision problems. In this article, we’ll break down the science of oxidative stress, its connection to cataractogenesis, and the exciting potential for antioxidant therapies that might one day change how we prevent and manage cataracts.
What Is Oxidative Stress, and Why Should You Care?
Let’s start with the basics. Oxidative stress refers to an imbalance between the production of reactive oxygen species and the body’s ability to detoxify them or repair the resulting damage. Think of ROS as the exhaust fumes of cellular metabolism. Your cells generate them naturally as they use oxygen to produce energy. But when these molecules build up without adequate control, they can damage proteins, lipids, and DNA.
Your eyes, and specifically your lenses, are particularly vulnerable to oxidative stress. Why? Because the lens is avascular—meaning it doesn’t have a direct blood supply—and relies on diffusion for nutrients and antioxidant protection. Over time, if ROS outpace the eye’s defence mechanisms, the lens proteins start to degrade, misfold, or clump together. That’s when light can’t pass through as cleanly, and you begin to notice the tell-tale signs of a cataract.
So, while oxidative stress is a systemic issue, the eye is especially at risk. And that’s why researchers have focused so heavily on this link when trying to understand cataract development.
How Does the Lens Protect Itself Against ROS?
Now, the lens isn’t defenceless. It’s actually packed with natural antioxidants designed to neutralise ROS and keep the internal environment stable. Glutathione is the superstar here. This small molecule is abundant in the lens and plays a key role in detoxifying harmful species before they can do serious damage. Other helpers include ascorbic acid (vitamin C), catalase, and superoxide dismutase—each with their own unique role in maintaining lens clarity.
But these protective systems aren’t infallible. As we age, the levels of glutathione in the lens decline. And once that buffer starts to falter, the proteins in the lens—particularly the crystallins—become more susceptible to oxidative modifications. These changes can cause them to unfold, lose solubility, and aggregate. That’s the molecular beginning of lens opacification.
Interestingly, studies show that the centre of the lens (the nucleus) often has lower levels of antioxidants than the outer layers. This is part of the reason why nuclear sclerotic cataracts are so common in older adults. The very area that gets the least protection is also where the damage tends to accumulate first.
Crystallin Protein Damage: The Real Culprit Behind Cloudy Lenses
Crystallins are the proteins responsible for maintaining the transparency and refractive properties of the eye’s lens. These proteins are highly stable and are designed to last a lifetime, which is important because the lens itself has little regenerative capability. Unlike many tissues in the body, the cells in the central part of the lens are never replaced. This long-term stability is a strength—but also a vulnerability. Over decades, these proteins accumulate damage from light exposure, metabolic by-products, and most critically, oxidative stress.
Reactive oxygen species (ROS) can modify crystallins through a range of biochemical mechanisms. One common alteration is the oxidation of amino acid residues, which changes the protein’s electrical charge and folding behaviour. This disrupts the native conformation of the protein, causing it to misfold or clump together with other damaged crystallins. These clumps are less soluble and begin to scatter light rather than allowing it to pass through cleanly, leading to the haze that characterises cataracts. In other words, the physical transparency of the lens is compromised at the molecular level.
Oxidative stress doesn’t just cause random chaos—it produces a specific signature of damage that scientists can now identify. Proteomic analyses of human cataract lenses have revealed oxidation at key sites on crystallin proteins, particularly at methionine, tryptophan, and cysteine residues. These changes aren’t seen as often in clear lenses, strongly suggesting a direct link between oxidative stress and cataract formation. What’s more, these alterations often appear before visible opacification, which means we might one day use them as early biomarkers of cataract risk.
Because these protein changes are cumulative and irreversible, the key to preventing cataracts lies in protection rather than repair. Researchers are increasingly looking at how to bolster the eye’s natural antioxidant systems, especially in people at higher risk. By enhancing the eye’s internal defences—whether through topical treatments, nutritional supplements, or novel drug delivery systems—we may be able to protect crystallins from oxidative harm and delay the onset of cataracts without ever needing to intervene surgically.
Environmental and Lifestyle Factors That Amplify Oxidative Stress
Although oxidative stress naturally increases with age, environmental exposures and daily habits can dramatically accelerate this process. Ultraviolet (UV) radiation is perhaps the most well-known external factor. When UV rays enter the eye, they can cause photochemical reactions that generate ROS directly in the lens. These free radicals then go on to damage proteins, lipids, and other critical components. Continuous, unprotected exposure—especially in environments with strong sunlight or reflective surfaces like water and snow—can significantly speed up lens ageing.
This is why consistent use of UV-protective sunglasses can have long-term benefits for eye health. It’s not just about comfort or reducing glare—it’s about preventing biochemical damage at the cellular level. Yet many people underestimate this risk and skip sun protection altogether, particularly on overcast days or during winter. It’s worth noting that cumulative exposure, not just sunburn-level events, contributes to oxidative stress in the lens. Over decades, even low-level exposure adds up.
Smoking is another major factor that elevates oxidative stress systemically, and the eyes are no exception. Tobacco smoke is rich in oxidants and free radicals, and it depletes the body’s natural antioxidant reserves. In particular, it reduces levels of vitamin C and glutathione, two key defenders in the lens. Smokers are not only more likely to develop cataracts but also tend to develop them at an earlier age. The toxic compounds in cigarette smoke are thought to directly modify crystallins and accelerate their aggregation, making smoking one of the most modifiable cataract risk factors.
Lastly, diet plays an influential but often overlooked role. Diets that lack antioxidants—such as vitamin E, carotenoids, and zinc—reduce the body’s ability to fight oxidative damage. Individuals who consume low amounts of fresh fruits and vegetables may have weaker ocular defences over time. Conversely, people who eat a nutrient-rich diet tend to have better long-term lens clarity. While food alone isn’t a cure-all, it acts as a critical support system, helping to keep oxidative stress in check and providing raw materials the body needs to repair and defend itself.
The Genetic Angle: Are Some People More Vulnerable?
Not all eyes are created equal when it comes to resisting oxidative stress. For some people, their genetic makeup means their natural defences are slightly less effective. Variants in genes that produce antioxidant enzymes—such as glutathione peroxidase, catalase, and superoxide dismutase—can influence how well an individual can neutralise reactive oxygen species. These enzymes are the first line of defence against oxidative stress, so even slight deficiencies can lead to increased vulnerability over time.
Another key area of interest is the genes that code for crystallin proteins themselves. Some individuals may inherit slightly different crystallin isoforms that are structurally less stable or more prone to oxidation. These subtle differences don’t cause problems early in life, but as oxidative stress accumulates with age, these “weaker” crystallins may degrade faster or clump together more readily. In these cases, people might develop cataracts earlier than average, even with minimal environmental risk factors.

Mitochondrial DNA also plays a role. Because mitochondria are a primary source of ROS, mutations in mitochondrial DNA can tip the balance toward a pro-oxidant state. Interestingly, mitochondrial DNA is inherited maternally, which could explain why early-onset cataracts sometimes appear across generations on the mother’s side. In these cases, oxidative stress is not just a matter of lifestyle or ageing but a systemic issue tied to cellular energy metabolism.
And then there’s the impact of chronic diseases like diabetes. Elevated blood sugar leads to increased glycation and oxidative stress throughout the body, including in the eye. People with poorly managed diabetes are more likely to develop cataracts—and earlier. For them, oxidative stress is not just a background process but an active, ongoing threat. This highlights the need for tailored prevention strategies that take into account both genetic predisposition and metabolic health, helping us move toward a more personalised approach to cataract care.
What the Latest Studies Show About Antioxidant Therapies
Given all this, it’s no surprise that researchers are looking for ways to boost the eye’s antioxidant defences. One promising direction involves delivering glutathione precursors directly into the eye to restore the lens’s natural protective shield. Compounds like N-acetylcysteine (NAC) are being explored as potential prodrugs that can replenish glutathione stores when applied as eye drops.
Another avenue is the use of small molecule antioxidants like lipoic acid and vitamin E analogues. Animal studies have shown that certain formulations can reduce oxidative stress in the lens and delay cataract formation. However, results in humans have been more mixed, partly due to difficulties in achieving effective intraocular concentrations via non-invasive methods.
There’s also emerging research into nanocarrier systems that can deliver antioxidants directly to lens cells. These nanoparticles may help protect crystallins by penetrating the dense protein matrix of the lens and releasing antioxidants slowly over time. It’s still early days, but some trials have shown success in restoring protein solubility in oxidised lens samples.
Can Diet Really Help Delay Cataracts?
Many people ask if a healthy diet alone can prevent cataracts. While diet isn’t a miracle fix, it can make a real difference. Epidemiological studies suggest that people who eat a diet high in antioxidants—especially carotenoids like lutein and zeaxanthin—have a lower risk of cataract development.
These compounds are found in leafy greens like spinach and kale, as well as in egg yolks and corn. They’re known to accumulate in the lens and retina, where they absorb blue light and help neutralise free radicals. Regular intake over time appears to have a cumulative protective effect.

That said, no single food or supplement can replace a broad-based approach. Balanced nutrition, sun protection, and smoking cessation all work together to reduce oxidative stress and preserve lens clarity. So yes—what you eat can absolutely support your eye health, especially when combined with other protective measures.
What About Lanosterol and Other Experimental Therapies?
One of the most talked-about experimental treatments in recent years has been lanosterol-based eye drops. In early animal studies, lanosterol appeared to reverse lens opacity by helping crystallins return to a more soluble state. While these findings generated enormous excitement, human trials have not yet confirmed the same level of effectiveness.
Other novel compounds, like rosmarinic acid and certain flavonoids, are also being tested for their ability to reduce oxidative stress in the lens. The goal is not just to prevent cataracts but to potentially reverse early-stage opacification without the need for surgery.
Still, we’re not there yet. Most of these treatments remain in preclinical stages or early human trials. But the fact that they’re being explored at all is a testament to how much progress has been made in understanding the oxidative origins of cataracts.
Looking Ahead: The Future of Cataract Prevention
Right now, the only definitive treatment for cataracts is surgical removal. But that could change. As we learn more about oxidative stress and how it damages the lens, researchers are getting closer to developing preventive therapies.
In the future, we may see a combination of topical antioxidants, oral supplements, and personalised genetic screening that helps identify individuals at higher risk. There’s even discussion of using AI to analyse retinal images and blood markers to predict oxidative stress levels and cataract risk before symptoms begin.
Until then, the best strategy remains a proactive one: protect your eyes from UV light, avoid smoking, eat a nutrient-rich diet, and stay up-to-date with eye exams. You can’t completely eliminate oxidative stress—but you can certainly give your eyes a fighting chance.
Final Thoughts
Oxidative stress is more than a buzzword—it’s a major player in the development of cataracts. The good news is that science is catching up. From understanding how ROS attack lens proteins to testing ways to neutralise that damage, we’re on the verge of breakthroughs that could transform how cataracts are treated and even prevented.
So next time you reach for your sunglasses or add spinach to your lunch, remember: you’re doing more than looking after your health. You’re helping your eyes stay clear and focused for the years ahead. And with the pace of research today, you might just see a future where cataract surgery isn’t the only solution.
References
- Truscott, R.J.W. and Friedrich, M.G., 2019. The etiology of human age-related cataract. Proteomics Clinical Applications, 13(2), p.1800152. Available at: https://doi.org/10.1002/prca.201800152
- Michael, R. and Bron, A.J., 2011. The ageing lens and cataract: a model of normal and pathological ageing. Philosophical Transactions of the Royal Society B: Biological Sciences, 366(1568), pp.1278–1292. Available at: https://doi.org/10.1098/rstb.2010.0300
- Spector, A., 1995. Oxidative stress-induced cataract: mechanism of action. FASEB Journal, 9(12), pp.1173–1182.
- Beebe, D.C., Holekamp, N.M. and Siegfried, C.J., 2011. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Research, 44(3), pp.155–165. Available at: https://www.karger.com/Article/FullText/321716
- Taylor, A., 1993. Cataract: relationship between nutrition and oxidation. Journal of the American College of Nutrition, 12(2), pp.138–146. Available at: https://www.tandfonline.com/doi/abs/10.1080/07315724.1993.10718293